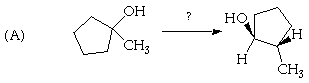
Final Exam Chem 3045x Wednesday, December 18 ,1996
Instructions: This is a closed book examination. You may not use any notes, books or external materials during the course of the examination. Please print your name and social security number on the front page of the examination. Be sure to allot your time in a manner that is related to the point value of the question. Be sure to show your reasoning wherever possible for partial credit.
All material to be graded must be on one of the pages of the exam with your name and social security number on the front page. If you need more space than is available on the page with the questions, use the back page of the previous page and label the number of the question on that page.
Time for the exam: three hours.
Total: 300 Points (15 questions 20 points each)
1. (20 Points) Suggest a plausible synthesis for the indicated compounds employing the indicated starting material and any inorganic or organic reagents you need in addition to the starting material. More than one step may be required for a plausible synthesis.
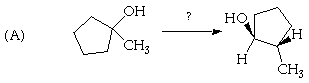
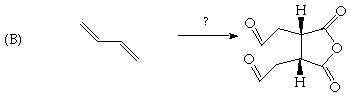
2. (20 Points) Suggest a plausible structure for C4H8 (A) and C10H16 (B) based on the indicated reactions. Be sure to indicate pertinent stereochemistry in the structures you suggest.
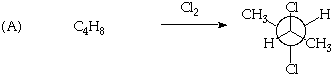
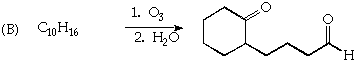
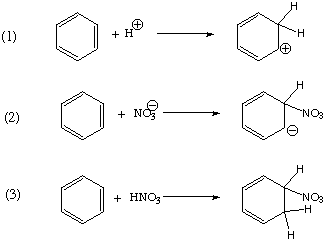
3. (20 Points) Under certain conditions, the nitration of benzene follows the rate law: rate = k[HNO3]. Which, if any, of the following is a possible rate determining step under these conditions? Explain your reasoning.
4. (20 Points) Three different dibromobenzenes (A, B and C) are known. Each is further brominated with Br2/FeBr3. Deduce the structures of A, B and C from the following information and explain your reasoning.
(1) A yields 2, and only 2, tribromobenzenes
(2) B yields 3, and only 3, tribromobenzenes
(3) C yields 1, and only a single, tribromobenzene
5. (20 Points) Identify the more stable stereoisomer in each of the following pairs and give the reason for your choice:
(a) cis-1-isopropyl-2-methylcyclohexane or trans-1-isopropyl-2- methylcyclohexane
(b) cis-1-isopropyl-3-methylcyclohexane or trans-1-isopropyl-3- methylcyclohexane
6. (20 Points) Which of the following compounds, if any, are chiral? Explain your reasoning for full credit.
(a) cis-1,2-dichlorocyclohexane (b) trans-1,2-dichlorocyclohexane
(c) cis-1,3-dichlorocyclohexane (d) trans-1,3-dichlorocyclohexane
7. (20 Points) Compound A (C7H13Br) is a tertiary bromide. On treatment with sodium ethoxide in ethanol, A is converted to a hydrocarbon B (C7H12). Ozonolysis of B gives C as the only product. Deduce the structures of A and B.
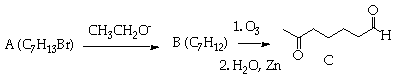
8. (20 Points) Which of the following bromides will undergo nucleophilic substitution more rapidly under SN1 conditions? Explain your reasoning.
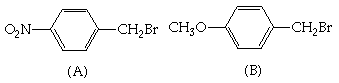
9. (20 points) Imagine molecules that existed in "Flatland", that is, in a two dimensional space. Translating the ideas of chirality in three dimensional space to two dimensional space, which, if any, of the following molecules are chiral in "Flatland"? Explain your reasoning.
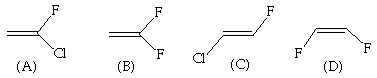
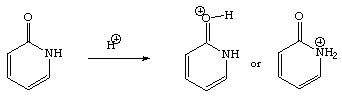
10. (20 Points) Employing Hückel theory and resonance theory, predict whether protonation will occur more favorably on the oxygen atom or the nitrogen atom?
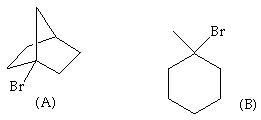
11. (20 Points) Consider the two tertiary bromides, A and B. One of these bromides is very reactive to elimination under basic conditions and is also reactive to nucleophilic substitution under acidic conditions,while the other is completely inert to both elimination and substitution under the same conditions. Which bromide is inert and why?
12. (20 Points) Treatment of the alcohol A with sulfuric acid yields the alkene B. Suggest a plausible mechanism for this reaction.
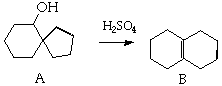
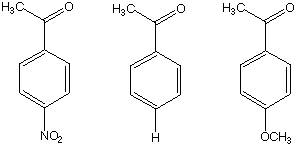
13. (20 Points) Consider that the infrared carbonyl stretching frequencies of the three compounds A, B, and C are quite different. Based on your knowledge of resonance theory and infrared spectroscopy, predict which will have the highest carbonyl stretching frequency and which will have the lowest carbonyl stretching frequency. Explain your reasoning.
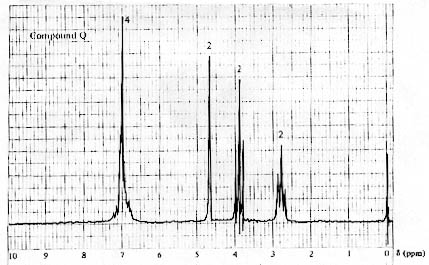
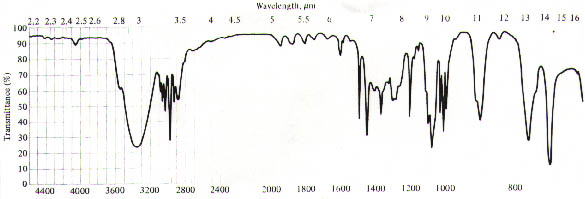
14. (20 Points) Suggest a plausible structure for a compound, C8H10O, possessing the IR and 1H NMR spectra shown below. The numbers next to the signals in the NMR spectrum correspond to the number of protons associated with the signals.
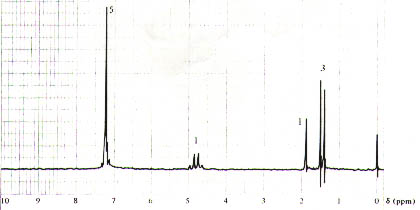
15. (20 Points). Suggest a plausible structure for a compound, C9H10O,possessing the 1H spectrum shown below. The numbers next to the signals correspond to the number of protons associated with the signals.