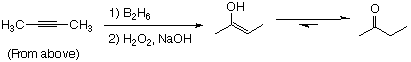
Exam 3 Chem 3045x Monday, November 9, 1996
Correlation tables for IR, 1H-NMR and 13C-NMR were attached on the last page of the exam. When you do this practice test, you may use the tables in the text for IR absorption freq., H-NMR chemical shifts, and C-NMR chemiscal shifts.
Time of the exam: 50 minutes.
Question 1: 10 points
Question 2: 10 points
Question 3: 20 points
Question 4: 20 points
Question 5: 30 points
Question 6: 10 Points
Total: 100 Points
NaI and acetone favors SN2 (See Text) Thus:
(a) 1-chlorohexane or cyclohexyl chloride
SN2 is faster with least steric hinderance. Thus 1° > 2° >> 3°
(b) 1-bromopentane or 1-fluoropentane
SN2 is faster with better leaving group. Thus I > Br > Cl > F
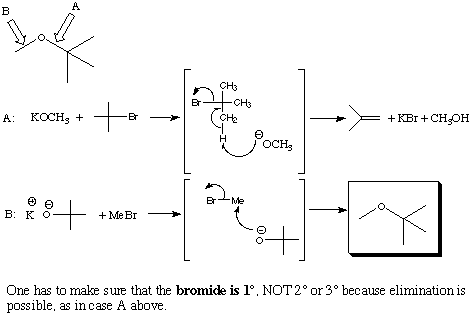
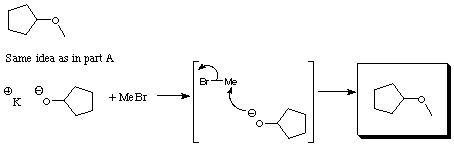
2.(10 Points) Under proper conditions alkyl bromides (RBr) react with potassium alkoxides (KOR') to yield ethers (ROR'). Suppose you which to prepare the two ethers A and B given below from an alkyl bromide and a potassium alkoxide. Select the combination of a specific alkylbromide and and a specific potassium alkoxide which would be most effective in preparing the following ethers. Explain the reason for your selection.
A

B
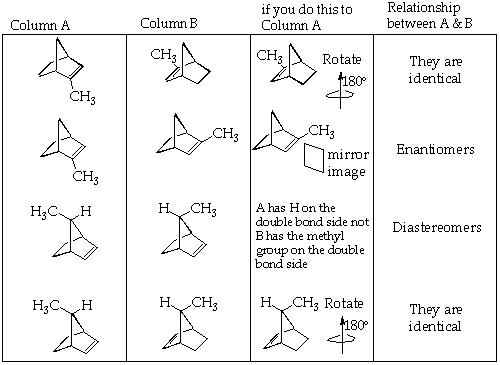
3.(20 points). Give the structural relationship between the following pairs as one of the following (a) constitutional isomers, enantiomers, diastereomers or identical.
4.(20 Points). Draw a Lewis structure consistent with the following NMR spectra and molecular compositions.
(a) Molecular composition C8H18; 1H-NMR consists of a singlet at d = 0.9 ppm
SODAR=0
(b) Molecular composition C8H8; 1H-NMR consists of a singlet at d = 5.8 ppm
SODAR=5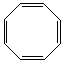
(c) Molecular composition C2H3Cl3; 1H-NMR consists of a singlet at d = 2.7 ppm
SODAR=0 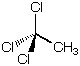
(d) Molecular composition C3H5Br; 13C-NMR consists of three signals at
d = 33 ppm (triplet), d = 118 ppm (triplet) and d = 134 ppm (doublet).
SODAR=1![]()
(e) Molecular composition C3H5Br; 13C-NMR spectrum consists of two signals at
d = 12 ppm (triplet) and 17 ppm (doublet).
SODAR=1![]()
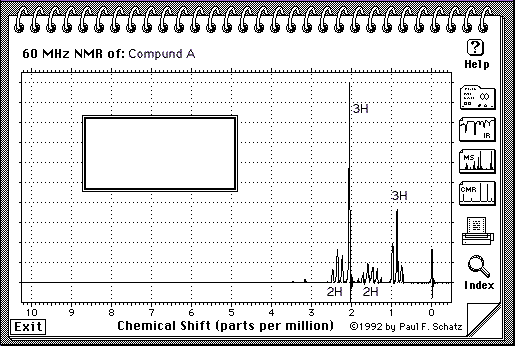
5.(30 Points. 10 Points for each spectrum) Suggest a structure that is consistent with the IR, 1H-NMR and 13C-NMR spectra shown on the following pages for the molecular compositions A = C5H10O, B = C6H10 and C = C5H12O. Indicate briefly how each structure is consistent with each spectra. The number of protons responsible for each signal is indicated under or next to the signal on the spectrum.
A = C5H10O
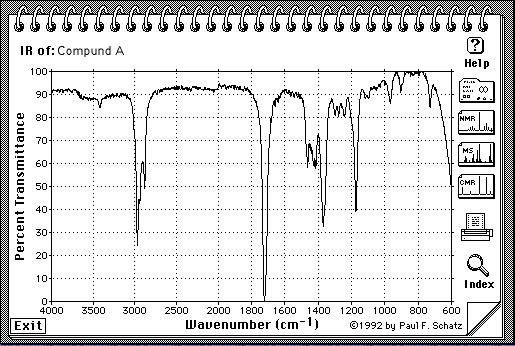
Note: H-NMR: The number of protons for each peak is given on the spectrum:
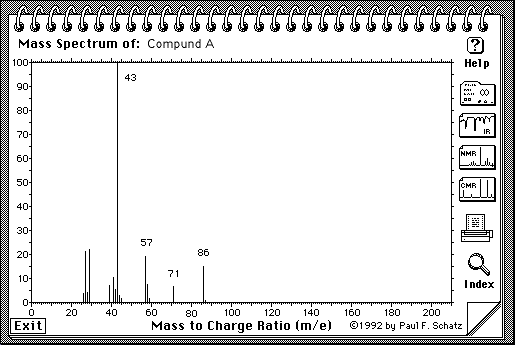
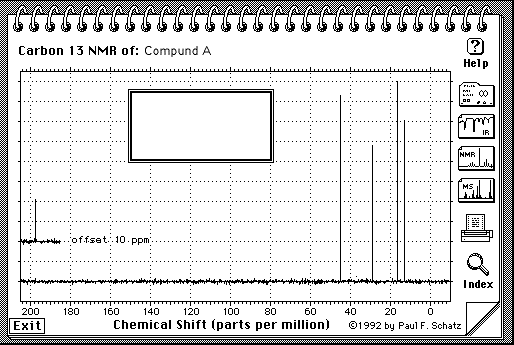
The suggested structure for A is
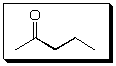
My reasoning for suggesting the structure for A:
IR: see C=O at ~ 1700cm-1
C-NMR: 5 different kinds of carbon, one of which is at about 210 ppm, which corresponds to C=O
H-NMR: See 2 terminal Me group (3H), one of which is a singlet (isolated), and the other one is a triplet (so there must be a methylene group next door). See also 2 two-H peaks, one is shifted further downfield and is triplet as well (so it has a methylene group next to itself and is closer to the C=O).
The suggested structure for B is
My reasoning for suggesting the structure of B is
I am sure that you can deduce the H-NMR and C-NMR. The alkene IR peaks are very helpful.
C = C5H12O
The suggested structure for C is
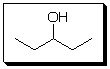
My reasoning for suggesting the structure of C is
Saturated compound with a big wide peak at 3300cm-1 suggests a saturated alcohol. The number of carbon peak in C-NMR is 3 while there are five carbons; symmetry allows only the structure drawn above.
6.(10 Points) From your knowledge of the theory of signal intensities in infrared spectroscopy, predict whether the symmetric or the antisymmetric stretch of carbon dioxide will be more intense in the infrared spectrum of this molecule and indicate your reasoning.
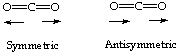
Symmetric stretches have minimal (if any) change in dipole moment; therefore the stretch on the left has VERY WEAK SIGNAL.
Asymmetric stretches have significant changes in dipole moment; therefore the stretch on the right is SIGNIFICANT.
EXAM IV (Question3-5 not included)
1. (10 Points). Consider each of the following structures. Which are Huckel aromatic compounds? Explain your reasoning for full credit.
Huckel's magic number for p electrons: 4n+2, where n=0, 1, 2, ...
All three molecules are therefore aromatic.
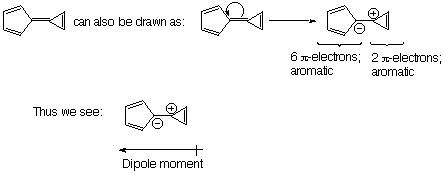
2. (10 Points). Use Huckel theory to explain why the following hydrocarbon possesses an unusually large dipole moment for a hydrocarbon. Which ring possesses the negative dipole and which possesses the positive dipole?
6. (30 Points). Write equations showing how you could prepare each of the following from benzene or toluene an any other necessary organic or inganic reagents. If an ortho and para mixture is formed in any step of your synthesis you may assum that the two isomers may be separated and that is not a problem in the overall synthesis.
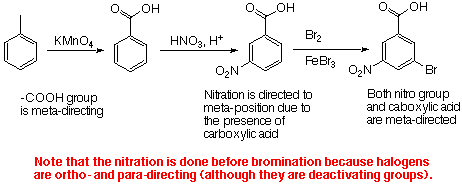
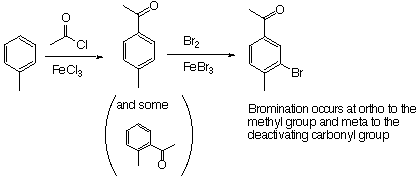
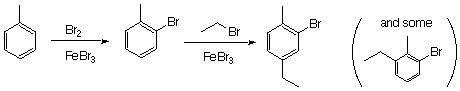
Note that if you were to reverse the two reaction, you will get competition between having the Br added ortho to the ethyl group and having it added ortho to the methyl group.
7. (20 Points). Starting with acetylene show how you would accomplish the following syntheses. You may use any organic or inorganic reagents in addition to acetylene.
![]()