Exam 2 Chem 3045x Friday, October 18, 1996
Instructions: This is a closed book examination. You may not use any notes, books or external materials during the course of the examination. Please print your name and social security number on the front page of the examination. Be sure to allot your time in a manner that is related to the point value of the question. Be sure to show your reasoning wherever possible for partial credit.
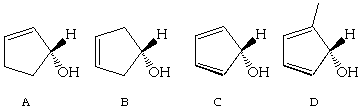
1 (10 Points) Which of the following structures are chiral? Indicate your reasoning for full credit.

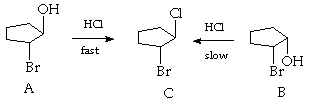
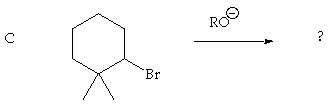
2. (10 Points) Treatment of either of the 1,2-bromoalcohols A or B with HCl results in formation of the 1-bromo-2-chloro cyclopentane C, but the rate of reaction of A to form C is much faster than the rate of reaction of B to form C. Suggest a plausible mechanistic interpretation of these results.
3. (30 Points) Predict the products of each of the following reactions. Give an indication of your thinking for full credit.
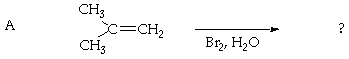
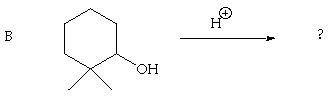
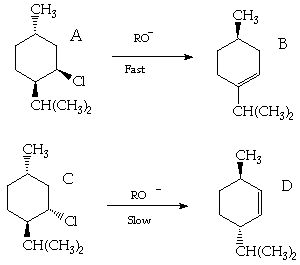
4. (20 Points). Treatment of neomenthyl chloride A with strong base results in a rapid reaction to produce B as the major product, while treatment of methyl chloride C results in a very slow reaction to produce D as the major product. Suggest a plausible mechanistic interpretation of these results. A chlorine atom possesses a smaller van der Waals diameter than a methyl group.
5. (20 Points. 5 Points each part) Suggest plausible syntheses, employing only known synthons, starting with the indicated organic molecules. More than one synthetic step may be required. You may use any inorganic reagents you require in any step.
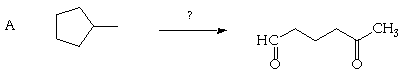
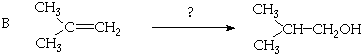
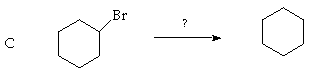
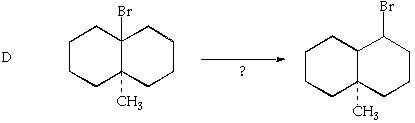
6. (10 Points. 5 Points each part). Based on analogy to reactions discussed in the text and lecture, what would you expect the major product of the following reactions? Indicate your reasoning for full credit.
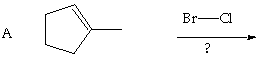
![]()