Exam 1 Chem 3045x Friday, September 27, 1996
Instructions: This is a closed book examination. You may not use any notes, books or external materials during the course of the examination. Print your name and social security identification number on the front page of the examination. Be sure to alot your time in a manner that is related to the point value of the question. All material to be graded must be on one of the pages of a copy of the exam with your name and social security number on the front page. There are two blank pages at the end of the exam if you need more space than is available on the page with the questions. Be sure to indicate the question you are answering if you use these back pages.
Time of the exam: 50 minutes.
Total: 100 Points
Point distribution:
|
Question 1: 5 points |
Question 4: 5 points |
Question 7: 20 Points |
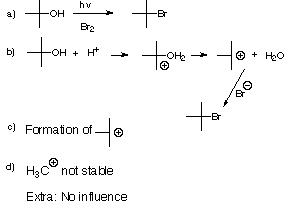
|
Question 2: 5 points |
Question 5: 10 points |
Question 8: 10 Points |
|
Question 3: 10 points |
Question 6: 15 Points |
Question 9: 20 Points |
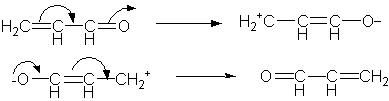
1. (5 Points). Use the curved arrow convention to show how both the following Lewis resonance structures can be interconverted.
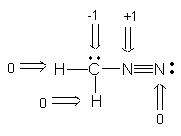
2. (5 Points). What are the formal charges ( 0, 1 or -1) on each of the atoms of the indicated Lewis structure? Place the formal charge next to each atom.
3. (10 Points). Consider the two isomers of composition C4H10, n-butane and iso-butane. One of these isomers boils at 0o C and the other boils at -10o C. Suggest a reason, based on intermolecular interactions, why one isomer boils at a different temperature than the other. According to your reasoning, which isomer boils at 0 oC.
n-butane: bp=0°C
iso-butane: bp=-10°C
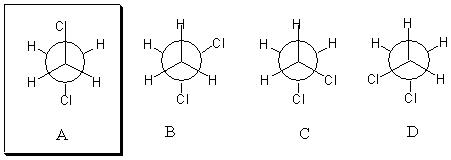
4. (5 Points). Circle the following structures which best represents the lowest energy conformation of 1,2-dichloroethane.
5. (10 Points). Select the compound from each of the following pairs that is converted more rapidly to the corresponding alkyl bromide on treatment with HBr. Indicate the reasoning for your choice.
(a) 1-butanol or 2-butanol
(b) 1-methylcyclopentanol or cyclohexanol
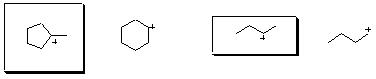
6. (15 Points). Consider the photochemical chlorination of each of the isomers possessing the composition C5H12 to produce monochlorides of composition C5H11Cl. Draw the Lewis structure of the isomer of composition C5H12 that produces
(a) a single monochloride
(b) three isomeric monochlorides

(c) four isomeric monochlorides

7. (20 Points) Menthol and neomenthol are constitutional isomers possessing the Lewis structure A. Menthol is the most stable stereoisomer possessing the constitution A and neomenthol is the next most stable stereoisomer possessing the Lewis structure A.
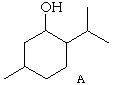
(a) Draw a structure which clearly shows the axial and equitorial positions of the substituents in menthol. (Hint: A hydroxy group is a smaller group than a methyl group.)
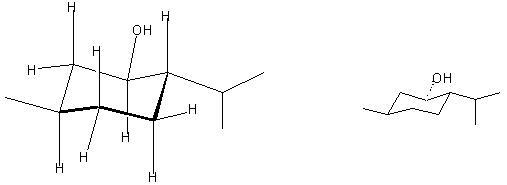
(b) Are the hydroxy and methyl groups cis or trans to each other in menthol?
Trans
(c) Draw a structure which clearly shows the axial and equitorial positions of the substituents in neomenthol.
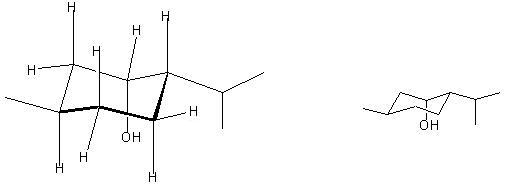
d) Are the hydroxy and methyl groups cis or trans to each other in neomenthol?
Cis
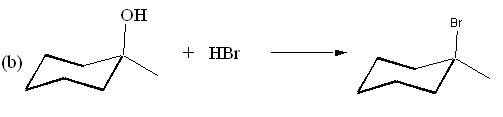
8. (10 Points) Draw the structure of the major product expected of each of the following reactions.
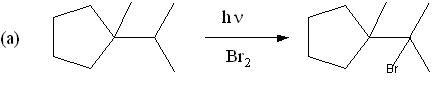
9. (20 Points). Consider the reaction of (CH3)3COH with HBr. (a) What is the major product of reaction? (b) Write out the elementary steps for the SN1 mechanism of reaction of (CH3)3COH with HBr. (c) Indicate which step in your mechanism is rate determining. (d) Based on your mechanism explain the following observation that CH3OH does not react with HBr under conditions for which the reaction of (CH3)3COH and HBr is complete in a few minutes. (5 points extra credit) How will the addition of a large amount of NaBr, a source of Br-, influence the rate of the reaction? Explain your reasoning.